Introduction
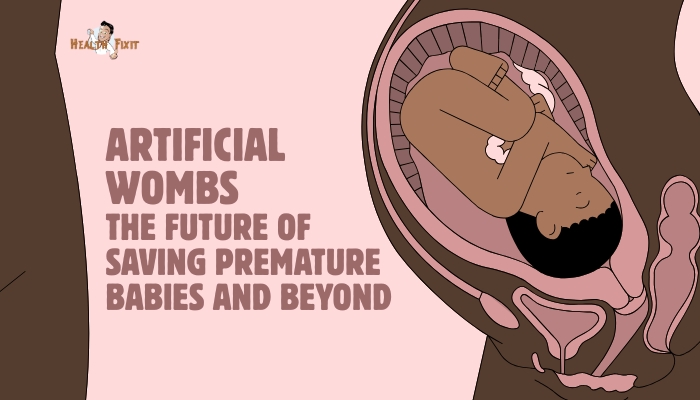
When extremely premature infants are born, their underdeveloped organs struggle to survive in the external environment.
Enter artificial wombs, sometimes called “bio-bags,” which aim to support these fragile neonates in a womb-like setup—providing amniotic fluid,
controlled gas exchange, and gentle physiologic support for continued development. Though still experimental,
progress suggests that artificial womb technology may someday revolutionize neonatal care, bridging the gap between the mother’s womb and independent life
. Looking even further ahead, this concept raises questions about whether entire gestations could one day occur outside the body, sparking both medical optimism and ethical debate.
Why Artificial Wombs?
The Challenge of Extreme Prematurity
Infants born before 28 weeks face high risks of respiratory failure, brain hemorrhage, and other complications.
Conventional incubators, mechanical ventilation, and IV nutrition help, but the environment remains fundamentally different from in utero conditions.
Despite medical advances, mortality and long-term disability rates remain significant for these extreme preterm neonates.
Mimicking the Womb
An artificial womb (ex vivo uterine environment) attempts to recreate:
- Amniotic Fluid: Submerging the fetus in a fluid that supports the lungs and prevents them from collapsing.
- Umbilical Cord–like Circulation: Oxygen and nutrients are supplied similarly to the natural placenta, bypassing the need for underdeveloped lungs to breathe atmospheric air.
- Gentle Conditions: Minimizing mechanical ventilation or harsh external stimuli, giving the infant’s organs more time to mature.
How Artificial Womb Technology Works
The “Bio-Bag” Prototype
In prominent animal experiments (e.g., lamb models at the Children’s Hospital of Philadelphia), researchers used a sterile, fluid-filled bag with an extracorporeal circuit. Key components:
- Amniotic Fluid Bath: A specialized fluid, close in composition to real amniotic fluid, fosters lung development.
- Oxygenation Circuit: Blood is pumped from the fetus through a low-resistance oxygenator, simulating the placenta’s oxygen exchange.
- Nutrient Delivery: Additional lines supply nutrients and remove waste, carefully controlling pH and electrolyte balance.
- Minimal Handling: The environment is dark and quiet, preserving a womb-like calm.
Key Innovations and Challenges
- Umbilical Cord Interface: Perfecting the connection that replaces the placenta is critical, ensuring stable pressure and preventing clotting.
- Sterility and Infection Control: The fetus is vulnerable to infection; thus the system must remain meticulously sealed.
- Regulating Growth Factors: The uterus secretes hormones and signals. Artificial setups must replicate or at least approximate these cues, so the baby’s organs mature properly.
Clinical Benefits and Goals
Lower Mortality and Morbidity
By shifting extremely preterm infants from harsh NICU interventions (like mechanical ventilation) to a more womb-like environment
, doctors hope to reduce lung injury, brain bleeds, and chronic ailments. Some studies in animals demonstrated better organ development than those in standard incubators.
Extended Window of Viability
Currently, the limit of viability is ~22–24 weeks. If an artificial womb can provide a supportive environment for these micro-preemies,
more babies might survive with fewer disabilities. This, in turn, prompts ethical questions around earlier viability thresholds.
Reduced Hospital Stays
With a more physiologic environment, experts predict fewer complications and shorter overall NICU stays, saving both emotional and financial costs for families and healthcare systems.
Challenges to Overcome
Scaling from Animals to Humans
While sheep fetuses have thrived in artificial womb prototypes, replicating success in human neonates is far more complex. Human physiology, immunology, and growth rates differ, and ethical constraints hamper direct clinical trials.
Regulation and Ethics
Moving from proof-of-concept to real-world therapy demands stringent FDA or equivalent approvals. The technology’s novelty raises concerns:
- Trial Protocols: Testing on critically ill preterm infants must balance potential benefit vs. risk.
- Long-Term Outcomes: We need data on survivors’ development—neurological, immune, etc.—over years or decades.
- Societal and Ethical Debates: If we can sustain a fetus outside the womb, does that alter the concept of pregnancy, parental roles, or viability definitions?
Complexity and Cost
Building and maintaining a sterile fluid environment, oxygenators, sensors, and real-time monitoring is expensive and technically challenging. Specialized training for staff is essential, meaning adoption might be limited to major centers initially.
Looking Ahead: Beyond Premature Infants
Full Gestation in a Lab?
Some envision a future where entire pregnancy might occur in an artificial womb, offering solutions for those with uterine constraints or certain medical risks. This remains speculative and highly controversial. The moral and legal frameworks would need major rethinking.
Research on Organ Development
Artificial wombs can also serve as labs to study fetal organ development or test maternal–fetal medication effects. This research might refine therapies for in utero conditions (like congenital heart anomalies) or even guide new regenerative medicine techniques.
Interplay with Genetic Engineering
In a world of advanced embryo screening or CRISPR-based genome editing, an artificial womb might theoretically “fine-tune” embryonic growth. However, combining these technologies raises intense ethical considerations around “designer babies” or manipulated gestation.
Practical Advice for Parents and Providers
- Timelines: Human clinical use is still on the horizon; current NICU standards remain gold standard for extremely preterm babies.
- Research and Trials: Families facing extremely preterm delivery should ask about any experimental protocols at specialized centers, though availability is extremely limited.
- Consult Specialists: High-risk obstetricians, neonatologists, and bioethicists can guide decisions. The moral and emotional aspects of possible future artificial womb therapies require careful counseling.
- Stay Informed: As labs refine the technology, new data on safety, short/long-term outcomes, and regulatory movement will shape if/when it becomes mainstream.
Conclusion
Artificial wombs hold remarkable promise for saving the most fragile neonates, bridging the gap between a mother’s womb and viability.
Although still in experimental phases, prototypes in animal trials have demonstrated improved organ growth and survival compared to traditional NICU methods.
If successfully translated to humans, this technology could redefine viability thresholds, reduce complications in extremely premature babies, and potentially shift the ethical and legal landscape of neonatal care.
Yet the road ahead is filled with hurdles: from perfecting an artificial placenta interface to ensuring robust safety data and grappling with the moral implications of sustaining early-stage fetuses ex utero. As research pushes forward
, the dream of “extreme preemies” thriving in protective “bio-bags” draws closer, hinting at a future where no baby is too small or too fragile to have a fighting chance at life.
References
- Partridge EA, Davey MG, Hornick MA, et al. An extra-uterine system to physiologically support the extreme premature lamb. Nat Commun. 2017;8:15112.
- Rinaldi L, Guglielmelli E, Mencagli G, et al. Artificial placenta-based systems for preterm infants: a review. Tissue Eng Part B Rev. 2021;27(4):277–289.
- Flake AW, Collins JJ, Van Meurs K, et al. The promise and challenges of artificial womb technology. Am J Obstet Gynecol. 2019;221(6):523–526.
- Usuda H, Watanabe S, Saito M, et al. Successful maintenance of key fetal physiologic parameters in preterm lambs treated by ex vivo uterine environment therapy. J Physiol Sci. 2017;67(3):337–347.
- Martecini S, et al. Ex-vivo fetal support: bridging the gap between fetal and neonatal care. Semin Fetal Neonatal Med. 2021;26(2):101210.
- Greenwood C, et al. Biobag: an artificial womb for preterm infants. Lancet. 2017;389(10085):1688–1689.
- Barkovich MB, et al. Ethical challenges: artificial womb technology and its potential effect on the abortion debate. Hastings Cent Rep. 2019;49(S3):S49–S58.
- Church GM, et al. On the horizon: gene editing and ex utero gestation. Nat Biotechnol. 2016;34(6):616–617.
- Alur P, Polin RA. The technology of next-generation neonatal intensive care. NeoReviews. 2020;21(8):e505–e516.
- Ross LF. Re-evaluating the significance of viability: artificial wombs and the next frontier in obstetrics. Am J Bioeth. 2021;21(2):4–6.